
Alumna Advances Innovation in Regulatory Affairs
July 29, 2025
 Regulatory affairs is one of the most impactful careers in life sciences. Professionals intersect science, policy, and innovation to bring effective medical products to the market.
Regulatory affairs is one of the most impactful careers in life sciences. Professionals intersect science, policy, and innovation to bring effective medical products to the market.
For Priyanka Dheer, M.S., Chapman University School of Pharmacy offered a uniquely supportive environment to explore regulatory affairs during her time as a Master’s student. Courses like Ethics, Regulation, and the Pharmaceutical Pipeline and Biopharma Regulatory Economics and Policy connected her technical knowledge to global healthcare systems, patient access, and commercial strategy.
Beyond coursework, rotations with pharmaceutical policy experts like Enrique Seoane-Vazquez, Ph.D., introduced her to trends facing cardiovascular devices, sparking an interest in the innovative opportunities in regulatory affairs. Another mentor, Marc Fleming, Ph.D., helped her design a capstone project that merged her interests in digital health and medical devices to study physician awareness of digital therapeutics in treating opioid use disorder.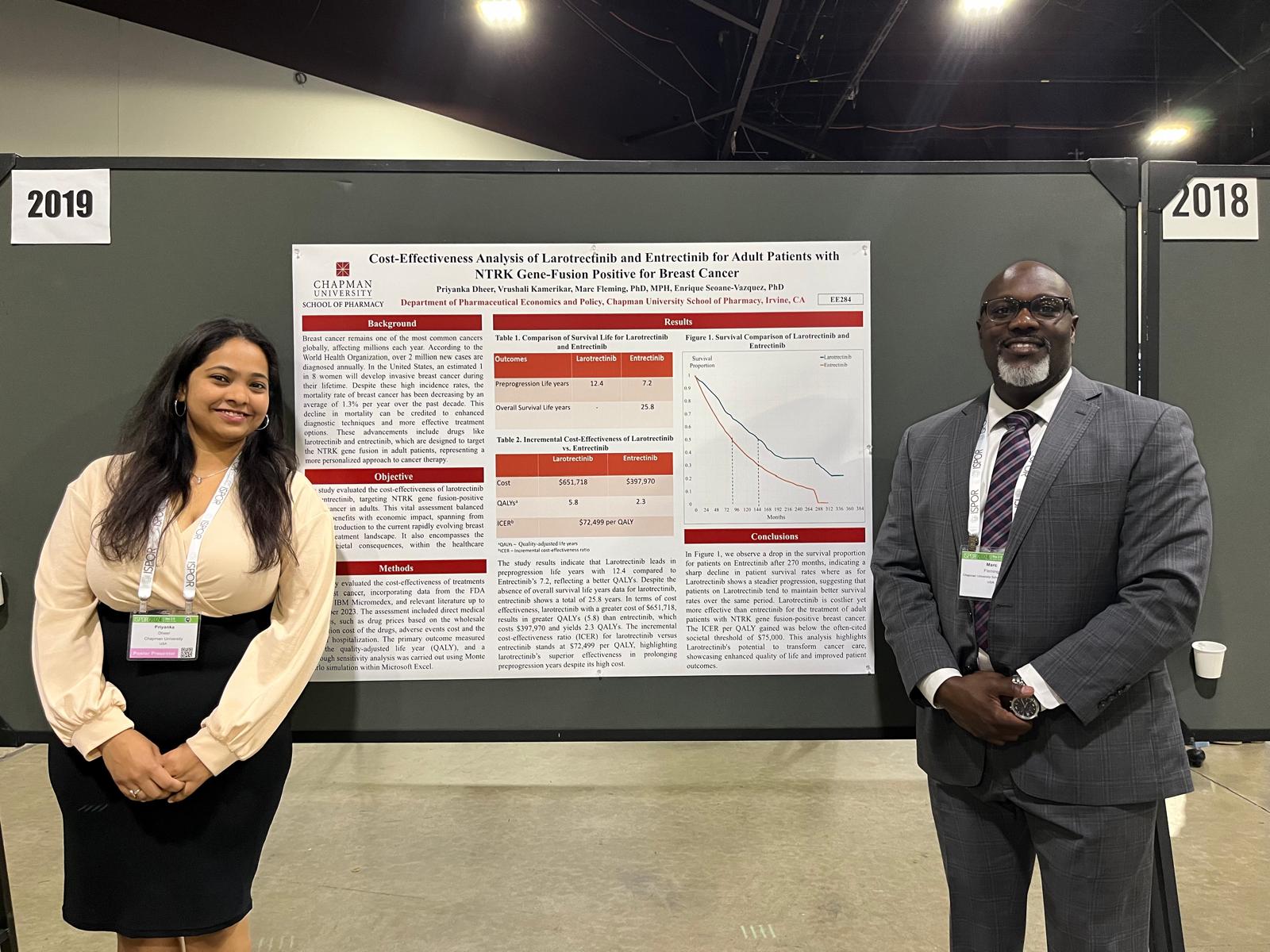
“It was incredibly motivating to work with a mentor who not only guided my academic development but also made space for my professional goals,” says Dheer. “Dr. Fleming took the time to understand my passion for medical devices and digital health and pushed me to consider opportunities for publication or presentation.”
Pursuing a Career in Regulatory Affairs
While Dheer graduated from CUSP in 2024 with her Master of Science in Pharmaceutical Sciences, she sees CUSP’s new Master of Science in Regulatory Affairs (M.S.R.A.) as a powerful opportunity for future professionals interested in the industry.

“The regulatory landscape is growing more complex and globalized, and employers increasingly seek professionals who not only understand the science behind products but also the regulations that govern them,” Dheer says. “A dedicated M.S.R.A. degree provides deeper and more structured exposure to essential areas like regulatory strategy, submissions, quality systems, and post-market surveillance, all skills that are directly applicable to real-world roles in pharma, biotech, and medical devices.”
She adds that the regulatory affairs program will add to what already makes Chapman University unique. “One of the standout aspects of Chapman University is the faculty’s deep connection to industry. Not only do they bring their own professional experience into the classroom, but they also frequently invite guest speakers from industry— regulatory professionals, quality experts, and leaders from pharma and medtech companies— who share real-world challenges and case studies.”
A Day in the Life
 At Hamilton Company, Dheer begins each day by reviewing updates from global and national agencies like the Food and Drug Administration as well as regulators in Europe, where the company’s products are shipped to. While she starts most days the same, she is ready for the new, unexpected challenges each day presents.
At Hamilton Company, Dheer begins each day by reviewing updates from global and national agencies like the Food and Drug Administration as well as regulators in Europe, where the company’s products are shipped to. While she starts most days the same, she is ready for the new, unexpected challenges each day presents.
“No two days are exactly alike, which makes the role both dynamic and intellectually engaging,” she says. Dheer is part translator, part strategist, and part detective as she collaborates with engineering, quality, and manufacturing teams to prepare regulatory standards that ensure compliance with international standards.
“The role demands a combination of big-picture thinking and meticulous attention to detail. It’s about bringing together science, engineering, and regulatory knowledge to support the delivery of safe, reliable, and high-quality robotic laboratory instruments to customers around the world.”
Building Career-Ready Skills
At Chapman, Dheer believes students are set-up for immediate success after graduation. As she reflects at her time at CUSP, Dheer recalls three major lessons that continue to serve her in her role at Hamilton Company.
- Teamwork matters: “It’s not just about dividing tasks, it’s about building trust, actively listening, and aligning on a common goal to produce high-quality outcomes.”
- Be adaptable: “Things don’t always go as planned—whether it’s shifting timelines, unexpected challenges, or new information, and being flexible and solution-oriented is crucial to moving forward.”
- Think big, act with precision: “It’s important to see the broader purpose of a project or decision, but at the same time, ensure that the execution is precise and thorough.”
Are you ready to take the next step in your career journey? Click here to learn about the MS in Regulatory Affairs program at Chapman University.

