CUSP Advances in Genetic Editing with CRISPR Technology
February 14, 2024
Offering new prospects for medical inventions, Chapman University School of Pharmacy researchers continue to make strides in gene editing. The recent study “Modifying peptide/lipid-associated nucleic acids (PLANAs) for CRISPR/Cas9 ribonucleoprotein delivery” centers around the manipulation of genetic material through the CRISPR technology, a technology that has regained momentum since initial reports in 2005. The authors, including faculty members Dr. Hamid Montazeri (Corresponding Author) and Dr. Keykavous Parang with PhD candidates Abdulelah Alhazza and Arthur Manda, focussed their research on using a recently introduced carrier for delivering the CRISPR technology to identify and address new tools to face the challenges in delivering specific genetic materials.
The core components in this genetic manipulation are Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated protein 9 (Cas9). CRISPR identifies a specific site on the genome and Cas9 works similarly to molecular scissors, facilitating precise modifications to genes. Early attempts encountered obstacles, particularly in the efficient delivery of CRISPR/Cas9 (similar to challenges faced in delivering small interfering RNAs or siRNA), directly into the cell nucleus, as the central repository of genetic information.
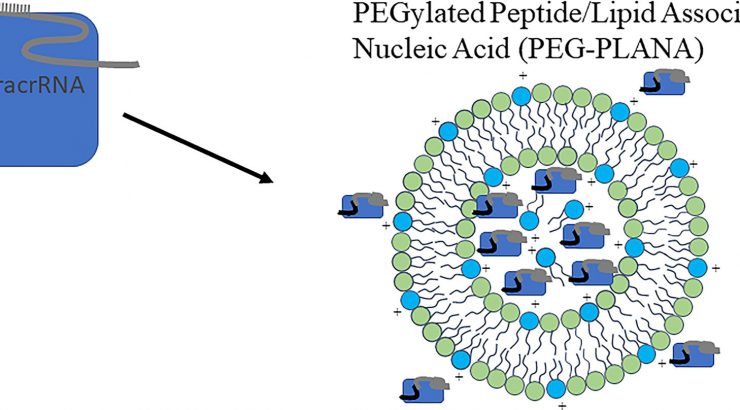
Recent breakthroughs have emerged from a previously reported project aimed at finding delivery systems termed Peptide/Lipid-Associated Nucleic Acids (PLANAs), which function as carriers for genetic materials. Initial experiments revealed an augmented level of toxicity under specific conditions.
In response to this challenge, the Chapman University team introduced polyethylene glycol (PEG) into the composition, which is recognized for its capacity to mitigate toxicity in delivery systems, acting as a protective shield for the carriers. The outcomes demonstrated a significant reduction in toxicity, meaning the carriers were “safe” in delivering the “cargo”.
“The CRISPR/Cas9 technology requires a compatible and efficient delivery system which was encouraging to investigate and evaluate using our lab novel delivery system (PLANAs),” says Abdulelah, one of the student authors and researchers. “It was exciting to see how cloning the cells confirmed the success of our delivery system to modify DNA sequence.”
The focus of the study extended to utilizing these enhanced carriers for the delivery of CRISPR/Cas9 gene-editing entities into cellular structures, and the results found efficient delivery mechanisms devoid of cellular harm, allowing for prospective medical applications. This advancement may lead to more dependable approaches to manipulating genetic code, potentially even leading to advanced medical treatments. The subsequent phase for these refined carriers involves comprehensive testing within living organisms, moving closer to practical and applicable real-world scenarios.